CARBOHYDRATES
Carbohydrates are one of the three macronutrients that make up the foundation of a healthy and balanced diet. They are an excellent source of energy for our bodies and are found in a wide range of foods, from fruits and vegetables to potatoes and rice. Despite their importance, carbohydrates have been the subject of much controversy and confusion in recent years, with many people advocating for low-carb diets or avoiding carbs altogether. However, the truth is that carbohydrates play a crucial role in energy production, muscle growth, strength, and overall health. In this long-form article, we will explore the different types of carbohydrates, their effects on the body, and how to incorporate them into a healthy diet to optimize performance, promote muscle growth and strength, and maintain good health.
Table of Contents
- What Are Carbohydrates?
- Carbohydrate Absorption & Digestion
- Intestinal Absorption of Glucose & Galactose
- Intestinal Absorption of Fructose
- The Glycemic Index (GI), Blood Sugar Spikes & Crashes
- Carbohydrates & Thermic Effect Of Food (TEF)
- Carbohydrates & Fat Oxidation
- Carbohydrates & Glycogen
- Glycogenesis & Glycogenolysis
- Glycogen & Exercise
- Carbohydrates & Leptin
- Summary
What Are Carbohydrates?
Carbohydrates are a class of organic molecules that are widely present on earth. They constitute the primary structural component of plants and serve as a source of energy in the form of starch and sugars. In humans, carbohydrates contribute to more than half of the energy consumed worldwide. Moreover, they act as metabolic intermediates, structural elements of cells and tissues, and constituents of RNA and DNA. The structural diversity of carbohydrates enables them to serve a wide range of functions in the body.
Carbohydrates are composed of carbon, oxygen, and hydrogen atoms in a proportion that resembles that of a “hydrate of carbon” (C-H2O)n, which is the reason behind the name “carbohydrate.” More specifically, carbohydrates are aldehydes or ketones with several hydroxyl groups.
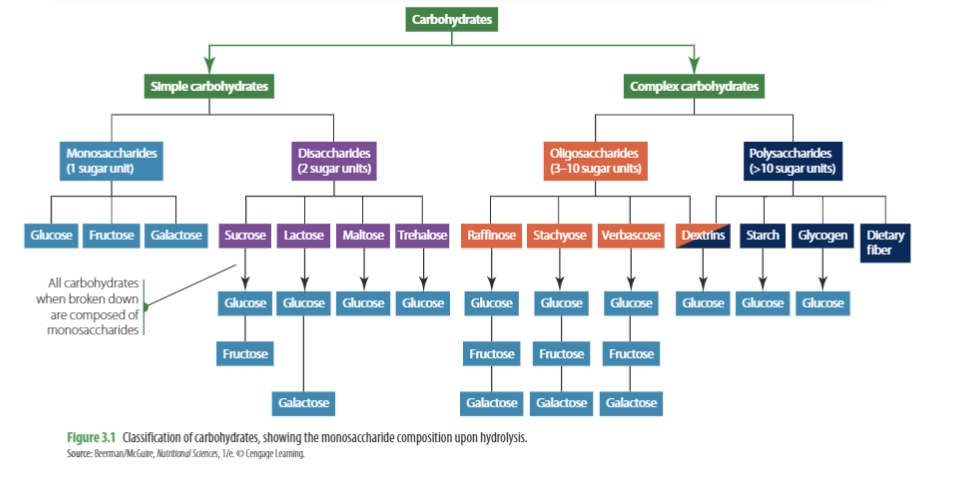
Carbohydrates are generally classified into two main groups: simple carbohydrates and complex carbohydrates. Simple carbohydrates are composed of one or two sugar molecules, also known as monosaccharides and disaccharides. Examples of simple carbohydrates include:
- glucose (dextrose)
- fructose (fruit sugar)
- lactose (milk sugar)
- maltose (hydrolyzed starch found in beer and malt beverages)
- sucrose (table sugar/cane sugar)
Complex carbohydrates can be further categorized into _oligosaccharides_ and _polysaccharides_. Oligosaccharides consist of 3-10 saccharide units and are found in foods such as beans, peas, bran, and whole grains. However, oligosaccharides cannot be easily digested by the body’s digestive enzymes, and are instead fermented by intestinal bacteria, which can cause flatulence.
Polysaccharides, on the other hand, consist of more than 10 saccharide units and include starch, glycogen, and cellulose. Starch, found in foods such as potatoes, cereal grains, legumes, and vegetables, is made up of two forms: amylose and amylopectin, both of which are polymers of glucose. Amylose makes up around 20% of the total starch content, while amylopectin accounts for approximately 80%. Glycogen is the body’s stored form of glucose, and cellulose is an important structural component of plant cell walls.
While it’s easy to categorize carbohydrates as “good” or “bad,” the distinction between simple and complex carbohydrates is arbitrary. However, this does not mean that all carbohydrates are equal when it comes to fat loss. For instance, many believe that sugar is more fattening than other carbs, but others argue that all carbs are eventually converted to glucose in the body. The truth is that different foods have varying effects on metabolism and absorption, and therefore, on body composition.
Unfortunately, many studies on carbohydrates do not control for other macronutrients or caloric intake, making it difficult to determine their effects on weight gain or loss. However, when comparing groups of people with identical characteristics, except for the source of carbohydrates in their diets, it becomes evident whether different types of carbohydrates have an impact on body composition. It’s important to note that while the body follows the laws of thermodynamics, eating excess calories leads to weight gain and eating fewer calories results in weight loss, regardless of the source of the calories. Therefore, the focus should be on studies that compare different types of carbohydrates in controlled settings rather than ad libitum protocols.
In a study conducted by researchers, participants were divided into two groups for six months. One group consumed a diet high in complex carbohydrates while the other group consumed a diet high in simple carbohydrates. Both diets contained the same total amount of calories and carbohydrates, which resulted in no significant differences in terms of fat loss, muscle retention, and effects on blood lipids.
Several other studies, have also found that varying sugar amounts in diets did not lead to differences in body composition changes. [1] Likewise, in another study, substituting complex carbs in a diet with simple carbs did not result in changes in body composition.
A recent systematic review found that replacing other iso-caloric carbohydrates with fructose does not lead to weight gain in humans. Contrary to popular belief, fructose is not more fattening than other carbohydrates when consumed in practical settings and in amounts less than 100 grams.
Furthermore, another study refutes the idea that fructose is more fattening than glucose. The study found that fructose does not cause more weight gain than glucose when both are consumed in equal quantities.
Main Takeaway: The composition of carbohydrates, whether simple or complex, does not have a significant impact on body composition or bodybuilding goals, as long as the total amount consumed is consistent.
In practical settings and when consumed in amounts below 100 grams, fructose is not considered to be more obesogenic than other carbohydrates. Furthermore, when consumed in equivalent quantities, fructose does not result in more weight gain than glucose.
Carbohydrate Absorption & Digestion
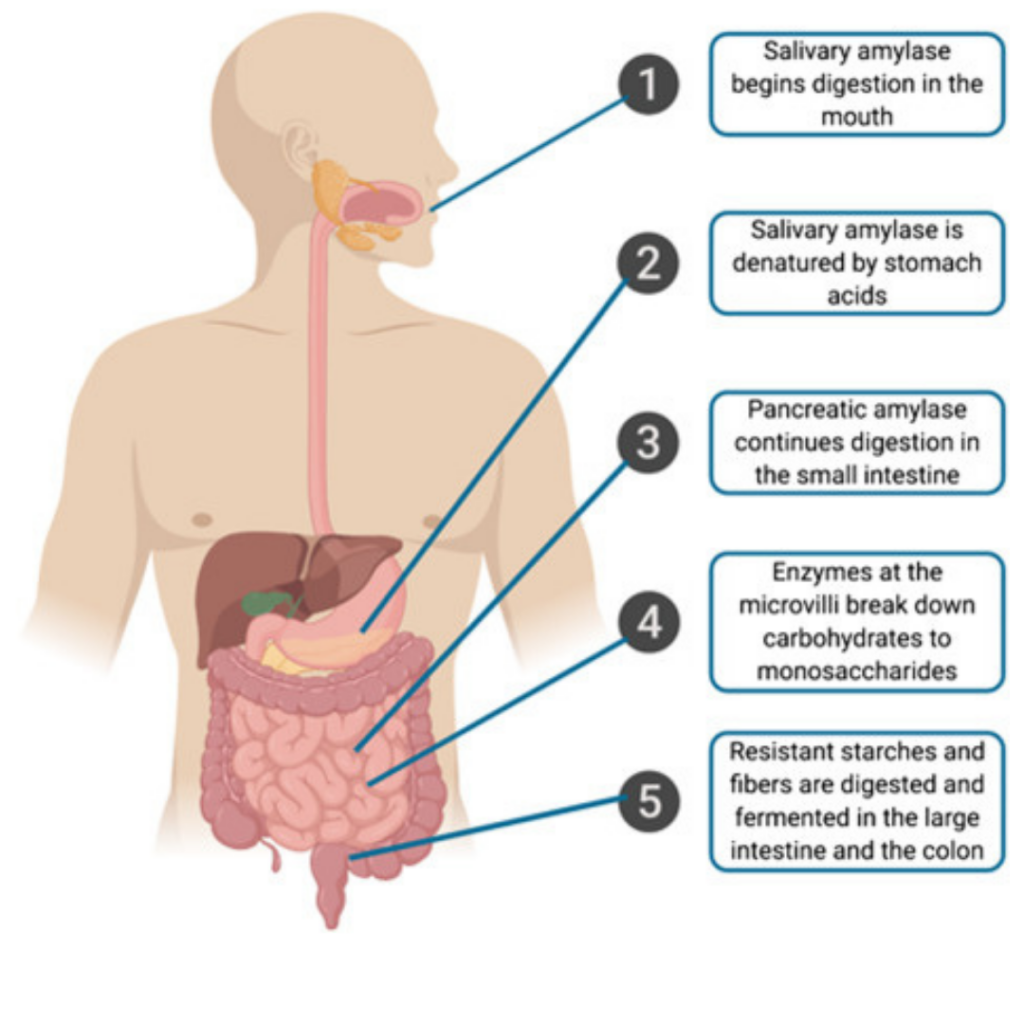
The process of digestion of dietary carbohydrates begins in the gastrointestinal (GI) tract, where they are hydrolyzed into monosaccharides by glycosidases or carbohydrases. Only monosaccharides can be absorbed by intestinal cells, while poly-, tri-, and disaccharides must be hydrolyzed first.
When glucose and fructose are present in food as monosaccharides, they require no digestion and can be absorbed directly by intestinal cells. However, the digestion of complex carbohydrates such as starches, amylose, and amylopectin begins in the mouth, where salivary a-amylase, a specific glycosidase, hydrolyzes a(1-4) glycosidic linkages. This phase of digestion mainly produces oligosaccharides (dextrins), but few mono or disaccharides due to the short time that food remains in the mouth before being swallowed.
Salivary a-amylase activity continues in the stomach until the pH is lowered by gastric acid, which inactivates the enzyme. It is important to note that a-amylase cannot hydrolyze the b(1-4) bonds of cellulose, the b(1-4) bonds of lactose, the a(1-2) bonds of sucrose, and the a(1-6) linkages that form branch points in amylopectin.
After the dextrins pass through the mouth, they enter the duodenum and jejunum, where pancreatic a-amylase acts on them. The pancreatic bicarbonate present in the duodenum raises the pH to an optimal level for enzymatic activity. Pancreatic a-amylase continues to break down a(1-4) glycosidic bonds to produce maltose, maltotriose, and limit dextrins. Maltotriose consists of three glucose units linked by a(1-4) bonds, while limit dextrins are remnants of branched amylopectin containing a(1-6) linkages that cannot be hydrolyzed by a-amylase.
Specific enzymes in the enterocyte brush border further digest the maltose, maltotriose, and limit dextrins. Maltose and maltotriose are hydrolyzed by a-glucosidase, also known as maltase, while limit dextrins are acted upon by a-limit dextrinase, also known as isomaltase. Only a-dextrinase is able to hydrolyze a(1-6) glycosidic bonds. Ultimately, glucose is produced as the final digestion product by the combined action of a-amylase and the brush border enzymes.
Certain types of starch found in beans, certain vegetables, and other foods with resistant starches are not completely broken down during digestion. This may be due to the limited accessibility of the food to the enzymes and the presence of natural inhibitors of a-amylase and a-glucosidase in some foods. As a result, enzyme inhibitors have been employed to slow down starch digestion for managing glycemic response.
The mouth, stomach, and lumen of the small intestine do not significantly digest disaccharides or small oligosaccharides. Disaccharide digestion mostly occurs within the brush border of the upper small intestine, via disaccharidase activity. The resulting monosaccharides are transported into the enterocytes using specific transporters. Enzymes such as lactase, sucrase, a-glucosidase (maltase), and trehalase are located on the enterocytes. Lactase specifically catalyzes the cleavage of lactose into equal amounts of galactose and glucose, and is stereospecific for the b(1-4) glycosidic bond in lactose.
In humans, lactase activity is highest during infancy but tends to decrease after weaning, which is also the case for most mammals. This reduction in lactase activity can result in lactose malabsorption and intolerance. The prevalence of lactose intolerance among human populations varies significantly based on factors such as race, ethnicity, and geographic location.
The enzymes sucrase, a-glucosidase (maltase), and trehalase are responsible for breaking down sucrose, maltose, and trehalose, respectively, into their respective monosaccharide components. Specifically, sucrase hydrolyzes sucrose into glucose and fructose, a-glucosidase (maltase) hydrolyzes maltose into two glucose units, and trehalase hydrolyzes trehalose into two glucose molecules. Once the carbohydrates are broken down into monosaccharides, they can be absorbed by the intestinal mucosal cells.
Nearly all dietary starches and disaccharides that can be absorbed are fully broken down into their individual monosaccharide components by specific glycosidases. Following this breakdown, the monosaccharides and residual disaccharides can be absorbed by the intestinal mucosal cells.
Intestinal Absorption of Glucose & Galactose
The intestinal brush border, composed of villi and microvilli, provides a large surface area for efficient nutrient absorption. The human intestine has a high absorptive capacity for glucose and fructose, estimated at 5,400 g/day and 4,800 g/day, respectively. This capacity is far beyond what is typically consumed in a normal diet. Carbohydrate digestion and absorption are highly efficient, with nearly all monosaccharides being absorbed by the end of the jejunum under normal conditions. Glucose and galactose enter enterocytes through both active transport (SGLTs) and facilitated transport (GLUTs).
- Glucose and galactose are absorbed into enterocytes through a mechanism that involves the sodium-glucose transporter 1 (SGLT1) receptor and requires cellular energy in the form of ATP.
- When there is a high concentration of glucose in the intestinal mucosa, glucose enters the enterocyte through the facilitated transporter type 2 (GLUT2). GLUT2 also facilitates the transport of glucose, galactose, and fructose out of the enterocyte, known as facilitated transport. This helps regulate intestinal glucose absorption and maintain stable blood sugar levels. GLUT2 is located selectively in the apical membrane of the enterocyte. However, in individuals with insulin resistance or type 2 diabetes, the receptor becomes resistant to the effect of insulin, leading to excessive absorption of glucose and elevated blood sugar levels.
Intestinal Absorption of Fructose
The absorption of dietary fructose occurs through facilitated diffusion and is mainly mediated by GLUT5, which has a high affinity for fructose and is not affected by glucose. Unlike glucose and galactose, fructose absorption does not require energy through SGLT1. The rate of fructose absorption is slower compared to glucose and galactose, but when GLUT2 is present in the brush border membrane of the enterocyte, the rate of absorption increases, as mentioned earlier.
GLUT2, the same transporter that moves glucose and galactose out of the cell, also transports fructose out of the enterocyte into the hepatic portal vein when its intracellular concentration increases. The facilitated transport process can only occur down a concentration gradient, from high to low concentration of fructose. At typical dietary intakes, fructose is not present beyond the portal vein. Fructose is efficiently absorbed by the liver, where it is phosphorylated. Fructose is absorbed entirely by facilitated diffusion, which makes its overall absorption rate slower than glucose or galactose, but faster than sugar alcohols such as sorbitol and xylitol, which are absorbed purely by passive diffusion.
A significant percentage of individuals (approximately 60%) are unable to fully absorb large quantities of fructose, usually ranging from 20 to 50 grams. This malabsorption leads to the influx of water into the intestine via osmosis, resulting in symptoms such as abdominal pain, flatulence, and diarrhea.
Once glucose, galactose, and fructose are absorbed in the intestine, they enter the hepatic portal vein and are transported directly to the liver. Almost all of the galactose and fructose are then metabolized and taken up by the liver through specific GLUT transporters.
The liver is able to convert both fructose and galactose to glucose derivatives, which can be stored as liver glycogen, released into the bloodstream to maintain normal blood glucose levels, or utilized for energy based on the liver’s energy requirements. Unlike glucose, little to no fructose and galactose are present in the peripheral blood, and their metabolism is not directly regulated by hormones in the same way as glucose homeostasis.
Glucose is the most abundant monosaccharide in nutrition because it is the main component of starch and is present in each of the three major disaccharides. The liver metabolizes glucose extensively, but it does not remove it as efficiently as fructose and galactose. The remaining glucose enters the systemic blood supply and is distributed among other tissues such as muscle, kidney, brain, and adipose tissue. Facilitated transport is responsible for glucose uptake in these organs, and it is insulin-dependent in skeletal muscle and adipose tissue, but insulin-independent in the liver, kidney, brain, and other tissues.
Fructose sources like orange and pomegranate juice, offer large amounts of antioxidants and vitamins, as well as lower cholesterol levels, and decreased levels of liver enzymes and body mass index (BMI).
Fruit intake is also associated with lower levels of liver fat, heart disease, cancer and mortality.
The Glycemic Index (GI), Blood Sugar Spikes & Crashes
Glucose is an essential source of energy for various cell types and plays a vital role in maintaining the normal functioning of bodily processes. Maintaining precise control over glucose concentration in the bloodstream is crucial for optimal health. Disruptions in glucose homeostasis can lead to the symptoms associated with diabetes mellitus.
While most dietary carbohydrates are ultimately converted to glucose in the bloodstream, the rate at which glucose appears in the blood can be influenced by the type of carbohydrate consumed.
The glycemic index (GI) is a tool used to measure the effect of carbohydrates on blood sugar levels after eating. It assigns a value between 0 to 100 to different types of carbohydrates based on how much they raise blood sugar levels. High GI foods are rapidly digested and absorbed, leading to sharp spikes in blood sugar levels. On the other hand, low GI foods are slowly digested and absorbed, resulting in gradual increases in blood sugar and insulin levels. However, it should be noted that there can be variations in GI values due to differences in methodology, such as the temperature of the food being consumed. The belief that table sugar causes a significant blood sugar spike followed by a crash due to its high GI is actually false.
The glycemic load (GL) is a measure that takes into account both the quality and quantity of carbohydrates in a food. It is calculated by multiplying the glycemic index of a food by the amount of carbohydrates in a typical serving of that food. The GL gives a more accurate representation of a food’s effect on blood sugar levels than the glycemic index alone, as it takes into account the amount of carbohydrate consumed.
Table sugar, due to its 50% fructose content, has a GI of ~68, which is a ‘medium’ effect on blood sugar. In fact, table sugar even has a lower GI than whole-wheat bread, which has a GI of ~72. The same thing applies to the insulin index.
The glycemic index of a diet does not determine its effects on body composition.
A study compared weight loss diets with identical energy content and macronutrient composition, but varying in glycemic index (and load), and found no differences in muscle retention or fat loss among groups. Health indicators, such as blood pressure, heart rate, fecal patterns, glucose and insulin metabolism, and blood lipids, were unaffected by the different diets. The only noticeable difference was a greater reduction in LDL cholesterol in the low-glycemic load group.
Similarly, these results were replicated in a study focused on weight gain instead of weight loss.
The maintenance of normal blood glucose levels is crucial for the body’s homeostasis, and it involves the collective metabolic processes of various organs such as the small intestine, liver, kidneys, skeletal muscle, and adipose tissue. This regulation is hormonally influenced and is primarily achieved through the antagonistic action of pancreatic hormones, insulin and glucagon, and to a lesser extent, the glucocorticoid hormones produced by the adrenal cortex.
The ingestion of carbohydrates leads to a rise in blood glucose levels, which in turn triggers the release of insulin and reduces the secretion of glucagon. It is worth noting that at any given moment, there are only around 4 grams of glucose in the blood. Insulin is the primary hormone responsible for lowering blood glucose levels and promoting anabolic processes. Its release stimulates the uptake of glucose, amino acids, and lipids by cells, leading to their conversion to storage forms in muscle and adipose tissue.
Glucose is stored in the form of glycogen through glycogenesis. The hormone glucagon, which has opposite effects to insulin, stimulates the breakdown of liver glycogen through glycogenolysis. In addition, glucocorticoid hormones, particularly cortisol, increase blood glucose levels by promoting hepatic gluconeogenesis.
Insulin and GLUT4 are crucial for the uptake of glucose in muscle and adipose tissue, particularly after consuming a meal high in carbohydrates. The series of events that involve insulin and GLUT4 are essential in regulating blood glucose levels and preventing hyperglycemia. When blood glucose levels increase, the b-cells of the pancreas release insulin into the bloodstream through exocytosis. Insulin then binds to specific insulin receptors on cell membranes of muscle and adipose tissue.
After insulin binds to specific receptors on the cell membranes of muscle and adipose tissue, GLUT4 translocates to the cell surface, where it can remove glucose from the blood. This process also triggers other important cellular responses. GLUT4 is an insulin-responsive transporter synthesized on the ribosomes of the rough endoplasmic reticulum and transferred to the Golgi apparatus, where it is packed into GLUT4 storage vesicles (GSVs). When insulin binds to its receptor, the GSV translocates to the cell membrane.
Studies show similar weight loss with widely varying levels of insulin and there is no evidence for high insulin causing weight gain. Weight gain and overeating causes high insulin, not the other way around.
According to a systematic review and meta-analysis, the impact of the glycemic index on health markers depends on the initial values of the markers. This suggests that low glycemic load diets are beneficial for individuals who are initially unhealthy, but have no effect on healthy individuals. Moreover, individuals who are more aerobically trained display a lower glycemic index response than those who are less trained.
This is a perfect example of the ceiling effect. If something isn’t broken, then you can’t fix it. So if you’re already healthy, eating _“healthy foods”_ at some point stops making you healthier. If you’re lean, you watch your diet and you’re physically active, then it’s safe to say you belong in the healthy category and the glycemic load of your diet has no considerable effect on your health.
When it comes to endurance exercise performance, ingestion of foods of different glycemic index 30 min prior to (one hour cycling) exercise does not result in significant changes in exercise performance, β-endorphin levels as well as carbohydrate and fat oxidation during exercise. It’s also important to note, that muscle tissue can utilise fructose as an energy source via glycolysis and glycogenesis, just not as a primary fuel substrate.
Main Takeaway: From the perspective of body composition, there is no significant difference in the effects of consuming low glycemic or high glycemic carbs, or simple versus complex carbs, if you are already healthy. The source of carbs is usually only relevant if you are unhealthy. Therefore, if you are already healthy, the type of carb you consume is generally not important when it comes to fat loss. In summary, when it comes to fat loss, all carbohydrates have a similar effect.
It is important to consider that various carbohydrate sources contain not only different macronutrients but also micronutrients and vitamins that are crucial for overall health. Therefore, it is not recommended to solely rely on tablespoons of sugar or candy as your main carb source. A diet rich in micronutrients and vitamins is best for overall health and adherence.
Carbohydrates & Thermic Effect Of Food (TEF)
The specific dynamic action (SDA), also known as the thermic effect of food (TEF) or dietary-induced thermogenesis (DIT), refers to the amount of energy used above the basal metabolic rate to process food for use and storage. Activation of brown adipose tissue following a meal also contributes to heat production and SDA. SDA is one of the components of metabolism, which also includes resting metabolic rate and exercise. Typically, SDA accounts for around 10% of one’s caloric intake, although the effect varies depending on the food components.
The thermic effect of food is the energy required for digestion, absorption, and disposal of ingested nutrients:
- Protein in foods has the greatest thermic effect, increasing energy expenditure 20–30%.
- Alcohol is a close second, with an energy expenditure of 20%.
- Carbohydrates have an intermediate effect, raising energy expenditure 5–10%,
- Fat increases energy expenditure 0–5%.
Carbohydrates & Fat Oxidation
Carbohydrates are rarely converted to fat, via a process called de novo lipogenesis, under normal dietary conditions. There are exceptions when this occurs. One is with massive chronic overfeeding of carbs, usually over 700-900 grams per day, for multiple days. Under those conditions, carbs max out glycogen stores, are in excess of total daily energy requirements and you see the conversion of carbohydrate to fat for storage. This however, is not a normal dietary situation for most people.
In general, the conversion of carbohydrates to fat for storage is not a significant pathway in humans. However, it’s important to note that excess carbohydrate consumption can contribute to fat gain indirectly. When you consume more carbohydrates than you burn, your body burns fewer fats for energy, which can increase the amount of dietary fat that is stored. Thus, while carbohydrates themselves may not be converted to fat for storage, consuming too many carbohydrates can lead to an increase in fat storage.
Carbohydrates do not lead to direct conversion and storage of fat. However, consuming excess carbohydrates can lead to fat gain by reducing the normal daily fat oxidation process, causing all consumed fat to be stored. This is why a surplus of 500 calories from fat and a surplus of 500 calories from carbohydrates can both contribute to weight gain.
The process of storing excess fat and excess carbs differs in the way they occur. When you consume 500 calories more of fat, it is stored directly, while with excess carbs, the increased carb oxidation decreases fat oxidation, resulting in more of the daily fat intake being stored as fat. The amount of carbs you consume affects how much carbs and fat your body burns.
Consuming more carbs leads to burning more carbs and less fat, while consuming fewer carbs results in burning less carbs and more fat.
This doesn’t mean that low carb diets are superior for fat loss.
There is an abundance of research showing that fat loss is not affected by the ratio of carbohydrates to fat in the diet [1], [2], [3], [4], [5], at least for sedentary individuals in non-ketogenic conditions and provided that protein and caloric intake are the same.
Overfeeding on carbohydrates results in just as much fat storage over time as overfeeding on fat when the total caloric intake is the same [1].
Carbohydrates & Glycogen
Glycogen is the major form of stored carbohydrate in animal tissues, located primarily in the liver (~100 g) and in skeletal muscle (~300-500 g normally). The glucose residues within glycogen serve as a readily available source of glucose. When dictated by the body’s energy demands, glucose residues are sequentially removed enzymatically from the glycogen chains and enter energy-releasing pathways of metabolism: glycogenolysis.
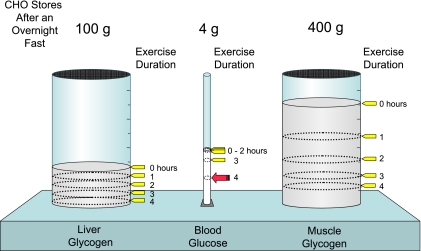
In a healthy, fasted 70-kg human, the liver and muscle can store approximately 100 g and 400 g of glycogen, respectively, while only 4 g of glucose is present in the blood. During exercise, glycogen reservoirs are depleted as glucose is preserved. The working muscles increase carbohydrate oxidation during exercise. After an hour of exercise, blood glucose levels are maintained, but at the expense of liver and muscle glycogen. Even after 2 hours of exercise, blood glucose levels can remain constant. Only during extremely prolonged exercise does blood glucose fall to levels low enough to cause severe neuroglycopenia.
Glucose cannot pass through the non-polar matrix of the lipid bilayer by simple diffusion as it is a highly polar molecule. Therefore, for glucose to enter cells, it needs to cross the cellular membrane, which requires active transport. In some absorptive cells, such as the epithelial cells of the small intestine and renal tubule, glucose is transported across the plasma membrane against the concentration gradient via a symport system (SGLT1) that pumps it with the help of an Na+/K+-ATPase.
A facilitated transport mechanism enables glucose to enter most cells in the body via specific transport proteins without the need for energy. These transporters are part of a family of carrier proteins known as glucose transporters or GLUTs, which are specific for glucose transport across cellular membranes in different tissues. The GLUT4 protein, which is responsible for glucose transport into adipose and muscle tissue, is stimulated by insulin. By translocating the pre-existing GLUT4 from intracellular vesicles to the cell membrane, insulin is necessary for the efficient and rapid transport of glucose into muscle cells.
Glycogenesis & Glycogenolysis
The term glycogenesis refers to the pathway by which glucose ultimately is converted into its storage form: glycogen. This process is vital to ensuring a reserve of quick energy.
The process of glycogenesis is especially significant in hepatocytes since the liver plays a key role in the synthesis and storage of glycogen. When needed, liver glycogen can be broken down into glucose and released into the bloodstream, helping to regulate blood glucose levels. The liver has the ability to both produce and lower blood glucose levels, even when insulin is not present, although insulin can induce the production of glucokinase, an enzyme that aids in glucose phosphorylation.
Skeletal muscle is another major site of glycogen storage in the body. While the concentration of glycogen in the liver is greater, muscle glycogen stores account for most of the body’s glycogen (about 75%) due to the greater muscle mass compared to the liver. Glycogen in muscle fibers serves as an energy source within the muscle cell and cannot directly contribute to blood glucose levels because muscle does not have the enzyme required to convert phosphorylated glucose back to free glucose.
The breakdown of glycogen into individual glucose units, in the form of glucose-1-phosphate, is called glycogenolysis and is catalyzed by the enzyme phosphorylase. Just like glycogenesis, glycogenolysis is highly regulated. The regulation is different for the phosphorylation isozymes in muscle than in liver, since the muscle and liver isozymes fulfill different physiological purposes.
- In muscle, the glucose is released from glycogen to provide glucose for energy within the cell.
- In the liver, glucose is released to provide blood glucose.
As phosphorylase (the catalyzing enzyme) is activated for glycogen phosphorylation, glycogen synthetase is inhibited.That means that the enzymes for glycogen synthesis and breakdown cannot be active at the same time.
Insulin is required for glucose to be transported into muscle cells, therefore making insulin production an important factor for maximum glycogen synthesis and muscle energy storage in order to be used during workouts. Since carbohydrate consumption stimulates insulin production, it has been theorized that you need to consume a lot of carbohydrates post-workout to help rebuild your glycogen stores.
Glycogen & Exercise
Even if a significant amount of glycogen is depleted, the body is very good at rapidly replenishing the fuel stores. Exercise causes translocation of GLUT4 from the GSVs to the cell membrane in your muscles to promote glucose uptake [1], just like the effect of insulin.
According to this study, individuals who performed leg extensions at 70% 1 RM until failure and then refrained from consuming anything afterward, showed a restoration of 75% of their glycogen levels within 6 hours. While the exercise protocol used in the study may seem unusual, it highlights the body’s remarkable ability to quickly replenish its energy stores.
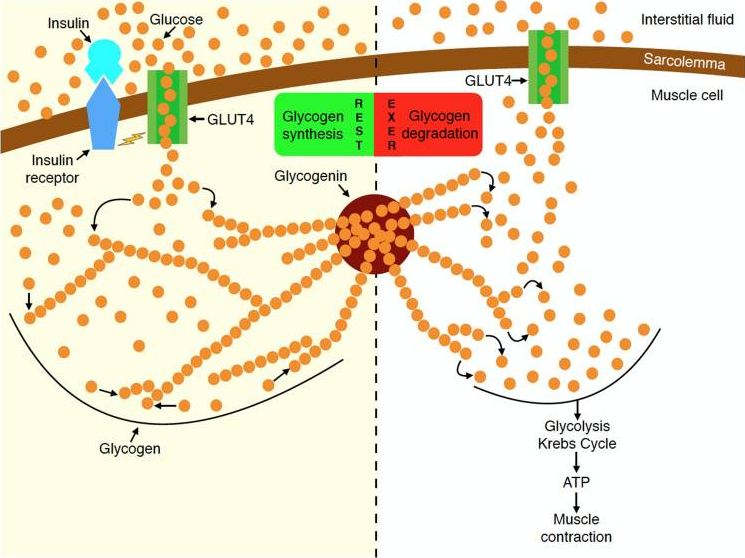
Depletion of muscle glycogen during exercise activates glycogen synthase, and this activation is greater when muscle glycogen is lower, resulting in a faster rate of glycogen resynthesis in the early post-exercise period. Following the cessation of exercise and with adequate carbohydrate consumption, muscle glycogen is rapidly resynthesised to near pre-exercise levels within 24 hours.
When rapid recovery of endogenous glycogen stores is a priority, ingesting glucose-fructose mixtures (or sucrose) at a rate of of ≥1.2 g carbohydrate per kg body mass per hour, can enhance glycogen repletion rates whilst also minimising gastrointestinal distress.
Main Takeaway: Most studies about glycogen depletion are performed on endurance athletes.
For the average Joe that lifts weights 3-4 times per week, full glycogen storage depletion will most likely never occur.
If you’re an athlete training multiple times per week however, proper nutrition is of extreme importance.
Carbohydrates & Leptin
Leptin is a hormone predominantly made by adipose cells and enterocytes in the small intestine that helps to regulate energy balance by inhibiting hunger, which in turn diminishes fat storage in adipocytes. Leptin acts on cell receptors in the arcuate and ventromedial nuclei, as well as other parts of the hypothalamus and dopaminergic neurons of the ventral tegmental area, consequently mediating feeding. In obesity, a decreased sensitivity to leptin occurs (similar to insulin resistance in type 2 diabetes), resulting in an inability to detect satiety despite high energy stores and high levels of leptin.
Leptin is controlled primarily by:
- Acute energy balance in the short term. A high calorie deficit causes leptin to drop lower than what can be explained by fat loss, and a caloric surplus raises leptin higher than what can be explained by fat gain.
- Total amount of fat mass in the long term. Fat cells are factories for leptin production. Not having many factories obviously impairs production and the aboslute amount of leptin in circulation.
Regular carb refeeds acutely increase leptin, while fat has no effect, proving it beneficial to circulating leptin levels during the diet.
Summary
Tldr; Regardless if the carbs you eat come from “healthy complex carbohydrates” or from cane sugar (glucose), both will inevitably end up as glucose just the same before they reach your blood stream. This is why from a fat loss standpoint, calorie per calorie, a carb is a carb, and starches and sugars are equally “fattening”.
You don’t need to worry about the glycemic index if you’re healthy. It is only when you’re not healthy and these homeostatic mechanisms do not function properly, that the GI of your food becomes relevant. Low glycemic load diets are good for your health if you’re initially unhealthy (like obese or diabetic), but in healthy populations there was no effect.
The composition of carbohydrates, whether simple or complex, does not have a significant impact on body composition or bodybuilding goals, as long as the total amount consumed is consistent.
In practical settings and when consumed in amounts below 100 grams, fructose is not considered to be more obesogenic than other carbohydrates. Furthermore, when consumed in equivalent quantities, fructose does not result in more weight gain than glucose.
It is important to consider that various carbohydrate sources contain not only different macronutrients but also micronutrients and vitamins that are crucial for overall health. Therefore, it is not recommended to solely rely on tablespoons of sugar or candy as your main carb source. A diet rich in micronutrients and vitamins is best for overall health and adherence.
Fructose is absolutely fine for consumption, and when combined with glucose will replenish glycogen storage levels at a very rapid pace post workout. Fructose sources like orange and pomegranate juice, offer large amounts of antioxidants and vitamins, as well as lower cholesterol levels, and decreased levels of liver enzymes and body mass index (BMI). Fruit intake is also associated with lower levels of liver fat, heart disease, cancer and mortality.
Carbohydrates are rarely converted to fat, via a process called de novo lipogenesis, under normal dietary conditions. Carbs don’t make you fat via direct conversion and storage to fat; but excess carbs can still make you fat by blunting out the normal daily fat oxidation so that all of the fat you’re eating is stored. Excess dietary carbs increases carb oxidation, impairing fat oxidation so more of your daily fat intake is stored as fat.
Insulin is required for glucose to be rapidly transported into muscle cells.
Most studies about glycogen depletion are performed on endurance athletes.
For the average Joe that lifts weights 3-4 times per week, full glycogen storage depletion will most likely never occur. If you’re an athlete training multiple times per week however, proper nutrition is of extreme importance.
Leptin is a hormone that helps to regulate energy balance by inhibiting hunger, which in turn diminishes fat storage in adipocytes. Regular carb refeeds acutely increase leptin, while fat has no effect, proving it beneficial to circulating leptin levels during the diet.







